- United States
Get non-surgical solutions for today's top aesthetic concerns with Venus Treatments. Join thousands of satisfied patients worldwide!
- Loading...
- All Regions
Get non-surgical solutions for today's top aesthetic concerns with Venus Treatments. Join thousands of satisfied patients worldwide!
Get the results you want with treatments that fit your life. Learn more about our safe, comfortable, and effective non-surgical aesthetic and hair restoration solutions today!
Search below to find a provider near you and to learn about our non-surgical aesthetic treatments with ARTAS®, NeoGraft®, Venus Bliss™, Venus Bliss MAX™, Venus Versa™, Venus Legacy™, Venus Velocity™, Venus Viva™ MD, and Venus Glow™.
Our success is your satisfaction. See why more and more patients worldwide are turning to Venus Concept non-surgical aesthetic treatments for all their aesthetic needs.
Over 10 million treatments performed worldwide every year
Patient satisfaction rate of over 90% for our key treatments*
Countries around the world offering Venus Concept treatments
Get started with our quiz to find the right treatments that can help achieve your aesthetic goals.
Explore our full range of aesthetic treatment options for today’s top concerns. Our certified providers perform the most in-demand face, body, and hair restoration procedures, ranging from cellulite reduction to facial rejuvenation, and skin resurfacing to fat treatments. Many of our non-invasive aesthetic treatments have been designed to work with even the busiest schedule. With most lasting 30 minutes of less, you can easily fit them into your lunchbreak, before or after work, or even on the weekends.
If you’re one of many who are looking for a solution to either reverse signs of aging, improve skin conditions, or target stubborn pockets of fat, non-invasive aesthetics treatments can help. Whichever non-surgical aesthetics treatment you choose, the visible results will leave you feeling great.

Take your anti-aging routine to the next level by fading your fine lines and wrinkles.
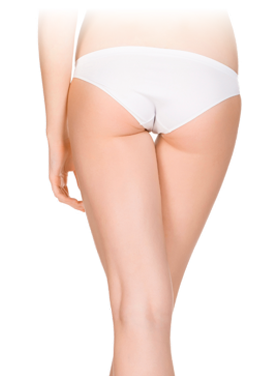
Target stubborn pockets of cellulite without surgery or downtime.
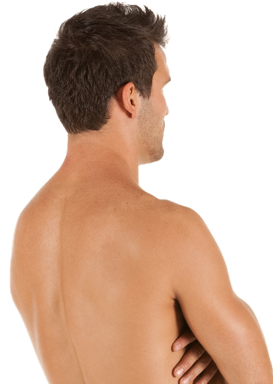
Get rid of unwanted hair on your face and body for irresistibly smooth skin.

Overcome hair loss with a minimally invasive solution that restores a fuller, natural-looking hairline

Get rid of acne-causing bacteria while healing inflammation from breakouts.

Diminish signs of premature skin aging and improve your overall skin tone.

Restore a brighter, healthier-looking complexion by deep-cleaning clogged pores.
Think you have to suffer through painful aesthetic procedures to get the results you want? Not with Venus Treatments. Each of our aesthetic and hair restoration devices are equipped with advanced technology to ensure patient safety and comfort. With state-of-the-art safety features, proven efficacy, and quick treatment sessions, we have a treatment solution to meet your needs. In some cases, just one of our non-invasive aesthetic procedures can be enough to attain your desired look. See for yourself by browsing our Before-And-After gallery below.
Quickly and easily clear acne and speed up the repair process for noticeably smoother, healthier-looking skin.
Devices that offer acne treatments: Venus Versa™
Results after: 6 treatments
Courtesy of Rosenberg Plastic Surgery
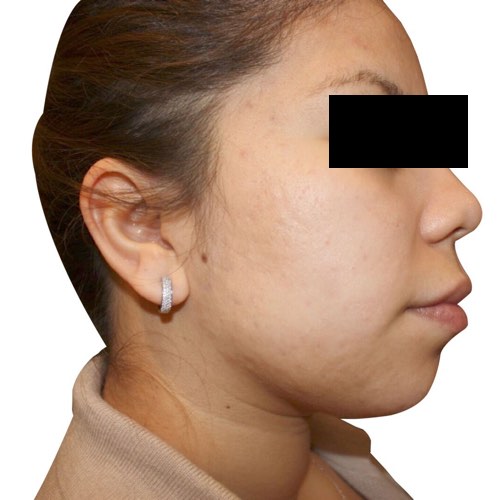 After
After
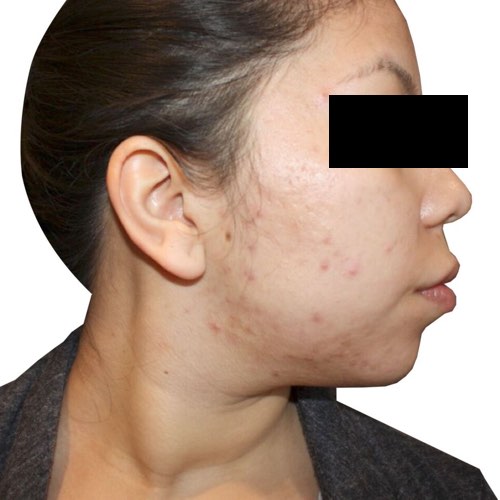 Before
BeforeShrink and fade the appearance of wrinkles for noticeably younger-looking skin.
Devices that offer wrinkle reduction: Venus Freeze Plus™, Venus Freeze™, Venus Legacy™, Venus Versa™, Venus Viva™
Results after: 6 treatments
Courtesy of Scott Callahan, MD
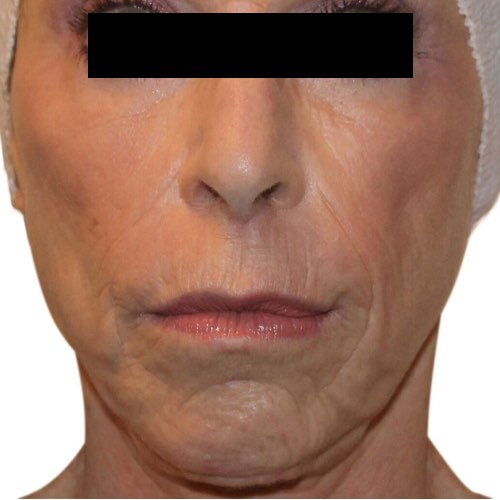 After
After
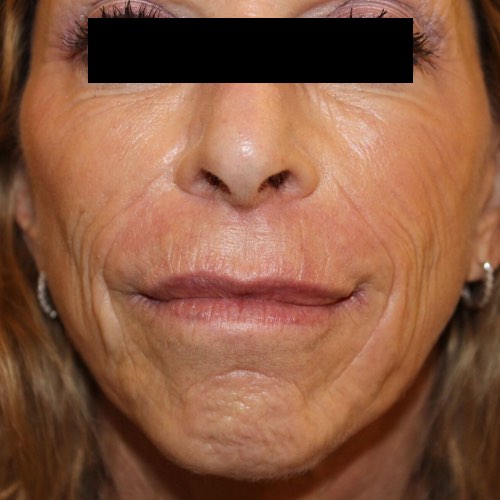 Before
BeforeFade the appearance of discoloration on the skin’s surface for a more even-toned complexion.
Devices that offer photofacial: Venus Versa™
Results after: 1 treatment
Courtesy of Tal Nachlieli, MD
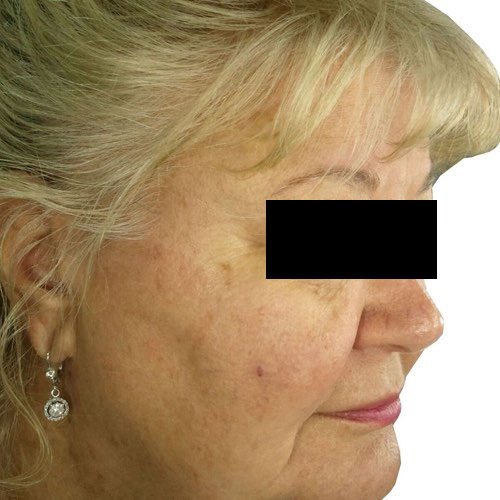 After
After
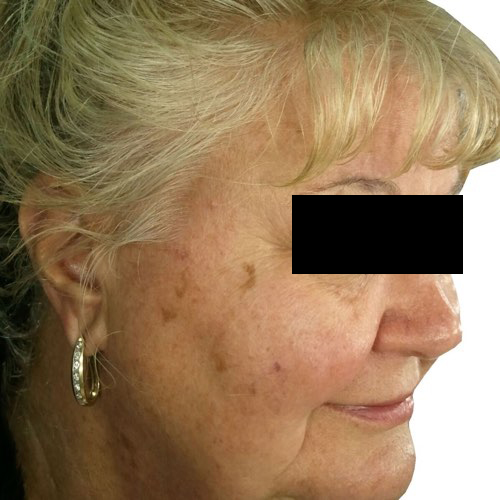 Before
BeforeTreatment technology is designed for quick hair removal in larger areas, like a man’s full back, so you get the results you want faster.
Devices that offer hair removal: Venus Velocity™, Venus Versa™
Results after: 2 treatments
Courtesy of Venus Concept
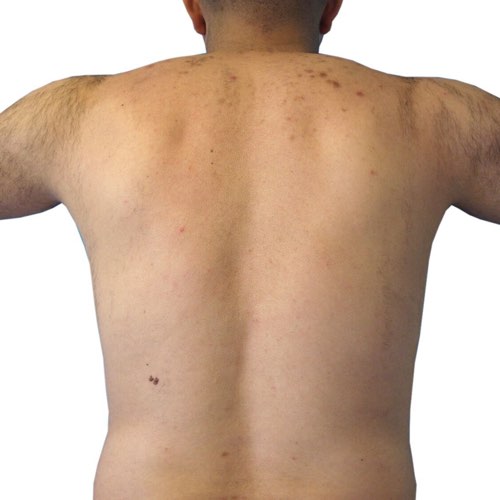 After
After
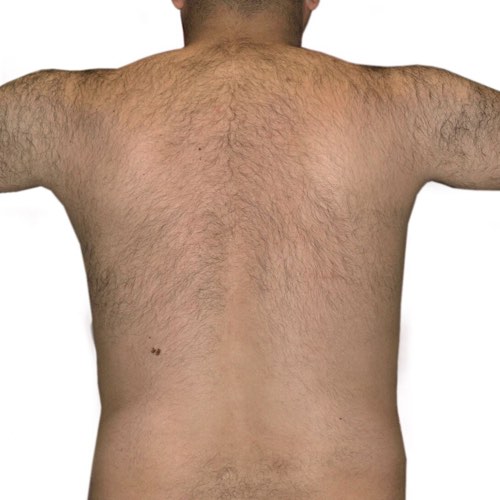 Before
BeforeDiminish acne scarring quickly and effectively after only a couple treatments.
Devices that offer acne scar reduction: Venus Viva™, Venus Versa™
Results after: 3 treatments
Courtesy of Neil Sadick, MD
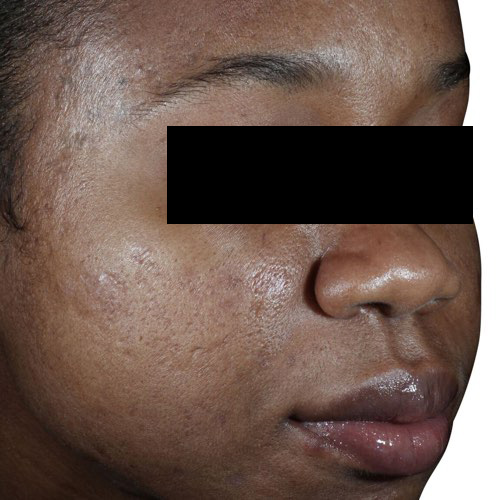 After
After
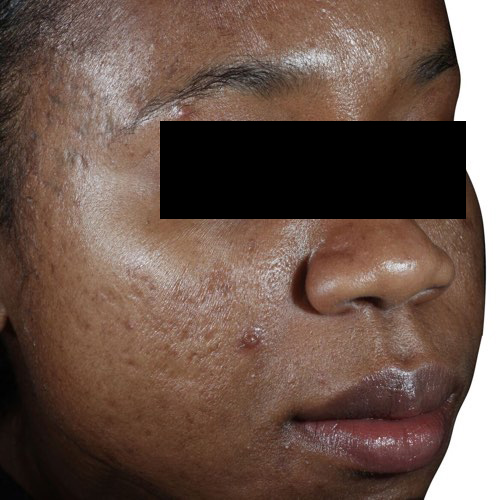 Before
BeforeReduce bumps and dimpling around the buttocks and thighs, resulting in a smoother, slimmer silhouette.
Devices that offer cellulite reduction: Venus Legacy™, Venus Versa™
Results after: 10 treatments
Courtesy of Synergy Aesthetics and Wellness
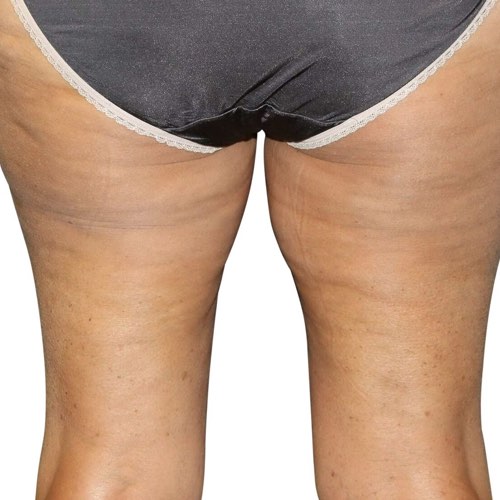 After
After
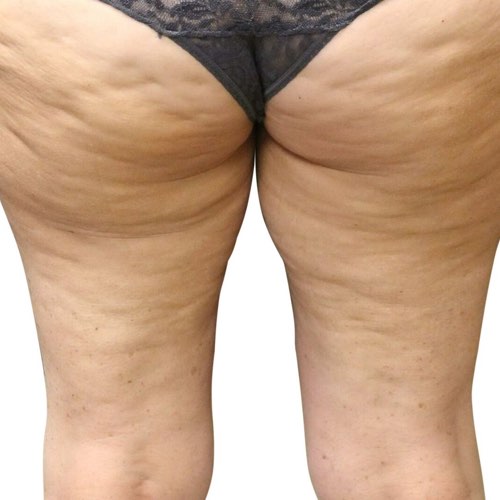 Before
BeforeTreatments work to break down stubborn fat cells around the abdomen, resulting in a slimmer-looking waistline.
Devices that offer body contouring: Venus Legacy™, Venus Versa™
Results after: 5 treatments
Courtesy of Beach Grove Laser
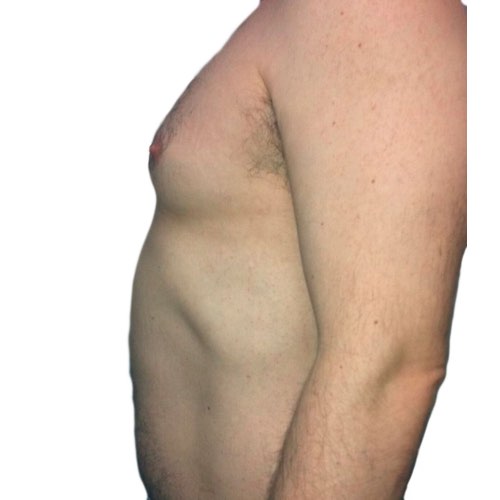 After
After
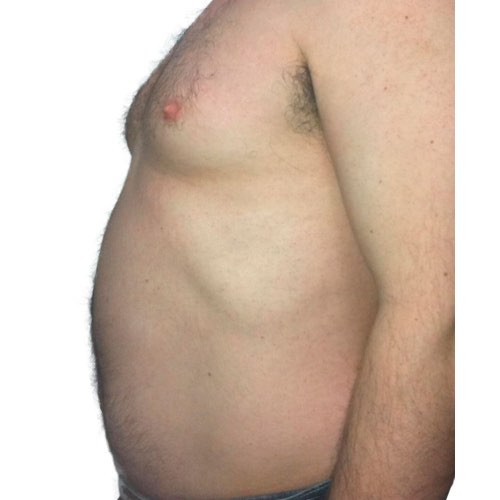 Before
BeforeThe Venus Treatments blog is your go-to source for tips, news, and information on the latest in beauty, skin care, fitness, and other lifestyle features.
Find a certified Venus Treatments provider near you today who specializes in today’s top aesthetic medical solutions.
For more information call: (888) 907-0115 // [email protected] // 4001 SW 47th Ave, Suite 206, Davie, Florida, 33314
REGULATORY CLEARANCES [ More ]
Venus Bliss™ is cleared by the FDA for non-invasive lipolysis of the abdomen and flanks in individuals with a Body Mass Index (BMI) of 30 or less, with the diode laser applicators. The (MP)2 applicator is cleared by the FDA for temporary reduction in the appearance of cellulite.
Venus Versa™ is cleared by the FDA as a multi-application device intended to be used in aesthetic and cosmetic procedures. The SR515 and SR580 applicators are cleared by the FDA for the treatment of benign pigmented epidermal and cutaneous lesions and treatment of benign cutaneous vascular lesions. The HR650/HR650XL and HR690/HR690XL applicators are cleared by the FDA, for the removal of unwanted hair and to effect stable long-term or permanent hair reduction for Fitzpatrick skin types I-IV. The AC Dual applicator is cleared by the FDA for the treatment of acne vulgaris. The DiamondPolar™ and OctiPolar™ applicators on the Venus Versa™ system are cleared by the FDA for non-invasive treatment of moderate to severe facial wrinkles and rhytides on females with Fitzpatrick skin types I-IV. The NanoFractional RF™ applicator is cleared by the FDA for dermatological procedures requiring ablation and resurfacing of the skin.
NeoGraft® is cleared by the FDA with indication for use in suction-assisted follicular extraction and re-implantation. NeoGraft® is an auto-graft system and can be used on both male and female patients.
ARTAS iX™ is cleared by the FDA with indication for use for harvesting hair follicles from the scalp in men diagnosed with androgenic alopecia (male pattern hair loss) who have black or brown straight hair. ARTAS iX™ is intended to assist physicians in identifying and extracting hair follicular units from the scalp during hair transplantation; creating recipient sites; and implanting harvested hair follicles.
Venus Legacy™ is cleared by the FDA for the non-invasive treatment of moderate to severe facial wrinkles and rhytides in females with Fitzpatrick Skin Types I-IV with the OctiPolar™ and DiamondPolar™ applicators, and temporary reduction in the appearance of cellulite with the 4D Body (LB2) and 4D Face (LF2) applicators.
Venus Velocity™ is cleared by the FDA for hair removal, permanent hair reduction (defined as the long-term stable reduction in the number of hairs re-growing when measured at 6, 9 and 12 months after the completion of a treatment regimen), and the treatment of pseudofolliculitis barbae for all Fitzpatrick skin types.
Venus Viva™ is cleared by the FDA for dermatological procedures requiring ablation and resurfacing of the skin. The DiamondPolar™ applicator is cleared by the FDA for the treatment of moderate to severe wrinkles and rhytides in Fitzpatrick skin types I-IV.
Venus Freeze Plus™ is cleared by the FDA for the non-invasive treatment of moderate to severe facial wrinkles and rhytides in Females with Fitzpatrick skin types I-IV.
Venus Heal™ is cleared by the FDA for the relief of minor muscle aches and pain, relief of muscle spasm, and temporary improvement of local blood circulation. These indications enable the treatment of certain soft tissue injuries and conditions.
Venus Freeze™ is cleared by the FDA for the non-invasive treatment of moderate to severe facial wrinkles and rhytides in Females with Fitzpatrick skin types I-IV.
Venus Glow™ is cleared by the FDA as a Class I motorized dermabrasion device. It provides a dermal rejuvenation treatment that works to open up and deep-clean pores. Venus Concept is the exclusive distributor for Venus Glow™.
Venus Swan™ is cleared by the FDA for the non-invasive treatment of moderate to severe facial wrinkles and rhytides.
Copyright © 2025 Venus Concept. All rights reserved.
You are entering our website. For other countries/regions and language options, please click the SELECT A DIFFERENT REGION button below.
SELECT A DIFFERENT REGIONAre you looking to get a treatment? Please visit our patient website to learn more.
Click HereUnsure which aesthetic treatment is right for you? Take this quick and easy quiz to discover treatments that suit your needs.
Get Started